How to Verify Massager Supplier Quality Beyond an ISO Certificate
- By Grace
- Updated on
You get a supplier's ISO 9001 certificate. It looks official, a reassuring stamp of approval that makes you feel like you've done your due diligence. Yet, six months later, you’re drowning in inconsistent product quality and rising customer complaints. So what went wrong?
That paper certificate is only the beginning of the story. A massager quality control system is only as good as its daily implementation on the factory floor. I’ve seen dozens of businesses get burned by suppliers who have the certification but lack the rigorous discipline to maintain standards batch after batch. At KLCOSY, we’ve built our reputation not on the certificates we hang on the wall, but on the transparent, auditable processes we practice every single day.
This guide will walk you through how to look beyond the paperwork and truly verify supplier quality. I'll show you the specific questions to ask and the evidence you should demand, giving you the tools to build a supply chain you can actually trust.
So, Why Isn't an ISO Certificate Enough to Guarantee Quality?
You did everything right. You chose a certified supplier to mitigate risk, but the problems are painfully familiar: defects, delays, and a frustrating lack of clear answers. That single piece of paper didn't protect your brand or your bottom line.
The hard truth is that an ISO certificate proves a quality management system exists on paper; it doesn't prove it's being followed. A sourcing manager for a major North American retailer told me, "Our last supplier was an ISO 9001 certified massager supplier, but when we had a field failure, they couldn't trace the component batch. We learned the hard way that a certificate without process discipline is meaningless."
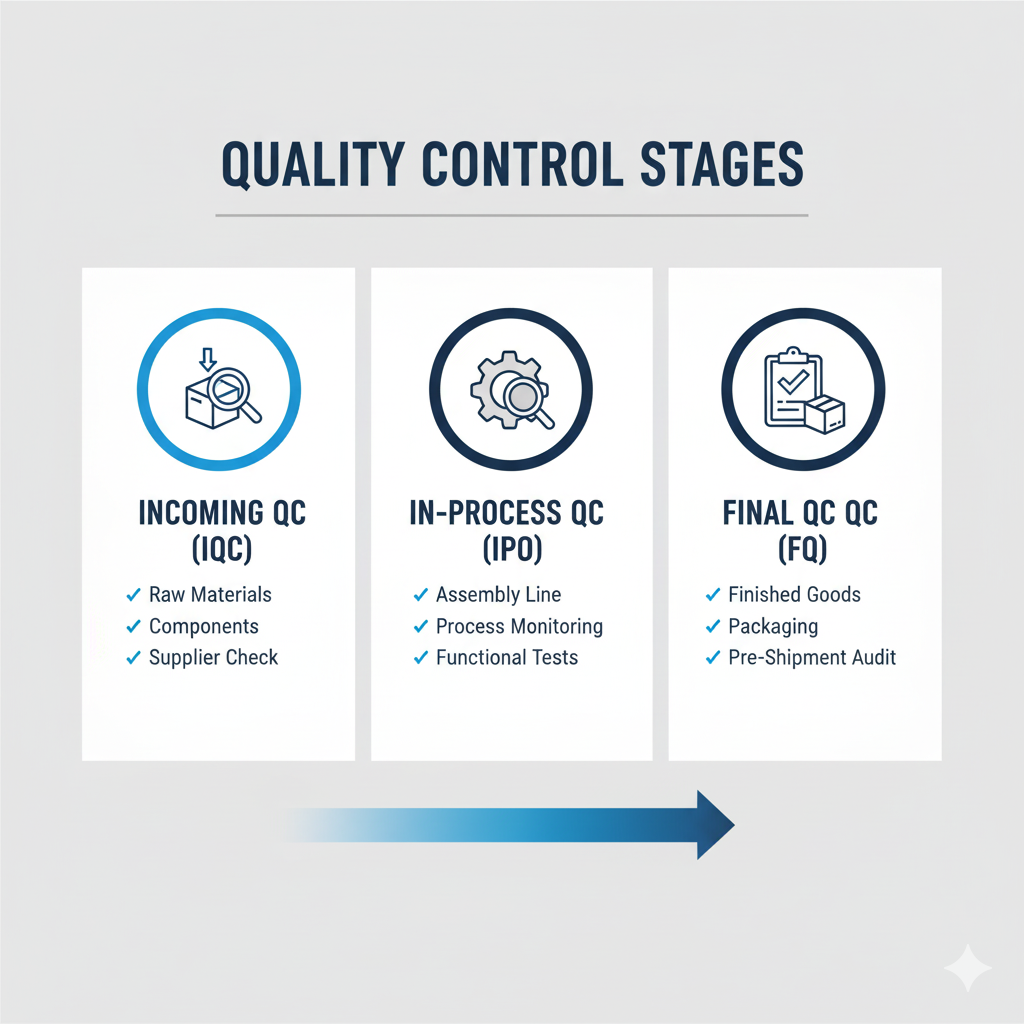
The real issue is the gap between "paper compliance" and "real-world discipline." Many factories can prepare the right documentation to pass an annual audit. But the true test of a quality system is what happens on a random Tuesday afternoon. Is every operator following the standard procedure? Are checks being documented accurately? An ISO certificate simply can’t answer these questions. That's why a hands-on massager supplier audit is critical for any serious buyer.
Paper Compliance vs. Real-World Discipline
A truly reliable partner can provide evidence of their discipline at every stage. They welcome your scrutiny because they are confident in their processes.

How to Verify Quality at the Source: The First Line of Defense
Your product's reliability depends entirely on the quality of its components. But how do you confirm your supplier isn't secretly cutting corners by using substandard raw materials to save a few cents?
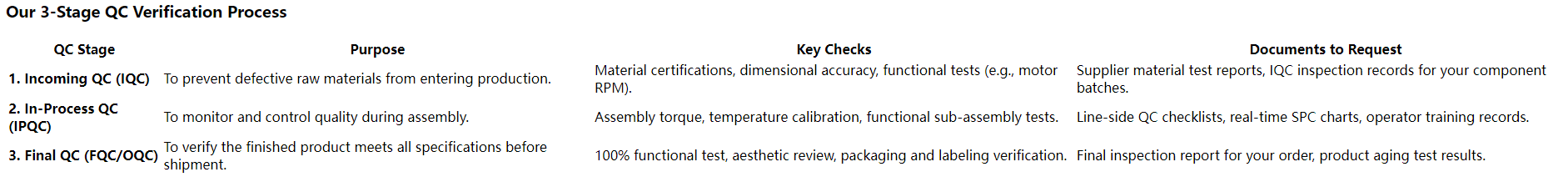
You must audit their Incoming Quality Control (IQC) for massagers. This is your first and most important line of defense against defects. A quality manager for a European medical device brand audited our IQC process and later told me, "Seeing your team meticulously test every batch of motors and graphene sheets before they even entered the warehouse gave us the confidence we needed. You prevent problems; you don't just find them later."

A robust IQC process is non-negotiable. It ensures every component meets the agreed-upon specs *before* it ever hits the assembly line. Every critical component, from the motor to the fabric, undergoes rigorous testing. Substandard materials are immediately rejected and sent back. Demanding to see detailed IQC reports for your specific component batches is one of the best ways to vet a new supplier.
How Do You Ensure Consistency from the First Unit to the Last?
The "golden sample" was perfect. But how do you know the 10,000th unit will be identical? This is where many supply chains fail, and where in-process and final controls become critical.
A buyer for a large promotional products company was impressed after reviewing our process. "The fact that you have a documented procedure for handling any line-side failure showed us you're serious about continuous improvement," she said. "We're not just buying a product; we're buying a reliable system."
Consistency is engineered, not accidental. Our In-Process Quality Control (IPQC) involves multiple check stations along the assembly line where key functions are tested. Then, every single finished product undergoes a 100% functional Final Quality Control (FQC) test before packaging. Nothing is left to chance.
Even the best systems can have issues. The true measure of a partner is how they respond. Our standard procedure is to immediately trigger a Corrective and Preventive Action (CAPA) process. We isolate the batch, find the root cause, and implement a fix to ensure the problem never happens again. This entire process is documented in a CAPA report, which is available for our clients to review. This transparency builds trust and proves our commitment to continuous improvement.
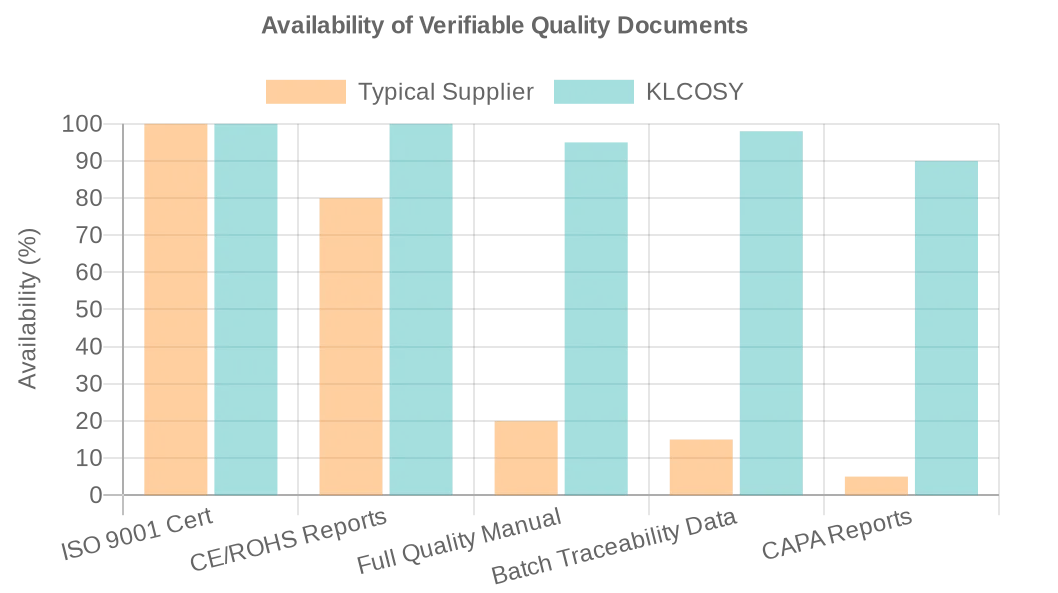
Verifying a supplier’s quality system goes far beyond asking for a certificate. It requires a commitment to auditing their processes, demanding evidence, and choosing a partner who embraces transparency. A manufacturer that is confident in its processes should welcome this scrutiny as an opportunity to build a strong, reliable partnership. We invite you to request our full certification package—including ISO 9001, CE, and ROHS—and schedule a virtual factory audit to see our quality control for massagers system in action.




